A new and very economical way to extract hydrogen fuel has been discovered by Tokyo University of Science in Japan. The set-up uses just a few basic ingredients – light from a mercury-xenon lamp, a solution of water and methanol, and a particular type of rust (or iron oxide) called α-FeOOH.
In the new study, researchers have outlined how this method yields 25 times more hydrogen (H2) than existing techniques that use titanium dioxide catalysts.
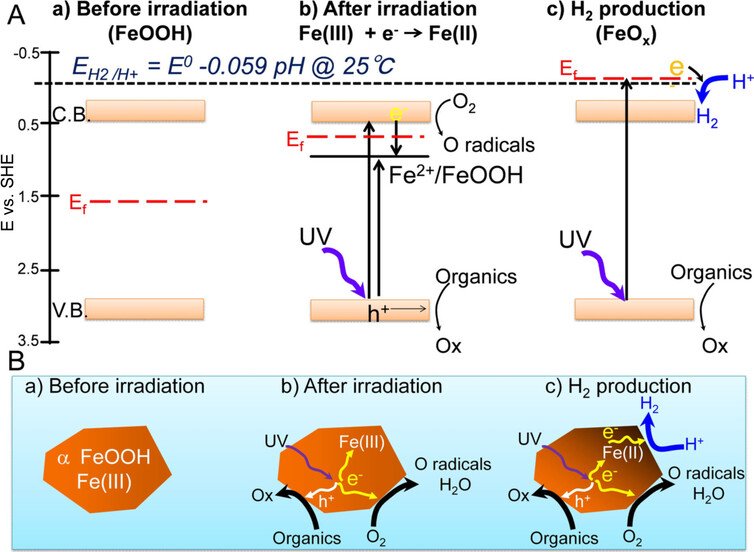
In the new method, by swapping titanium with rust, the H2 gas generated seemed to be blocked from recoupling with oxygen, making the separation of the elements easier, and reducing the risk of explosion at the same time.

The researchers were surprised at the generation of H2 using this catalyst, because most of the iron oxides are not known to reduce to hydrogen, as well as being more common (and therefore cheaper) than other metals used as catalysts to produce hydrogen, this type of rust also seems to be very stable too.
Considering the source for the hydrogen in this case is simple organic waste, the new approach could potentially make a huge difference to energy systems – a hydrogen production process that does more with less.
However, one of the areas the team wants to investigate next is why oxygen is so crucial to the production process (when it was removed from the catalyst, the experiments failed).
This latest study describes a significant step forward, but plenty more research is going to be required before we can power our cars with hydrogen.
Reference- Science Alert, Chemistry – A European Journal, Tokyo University of Science website






