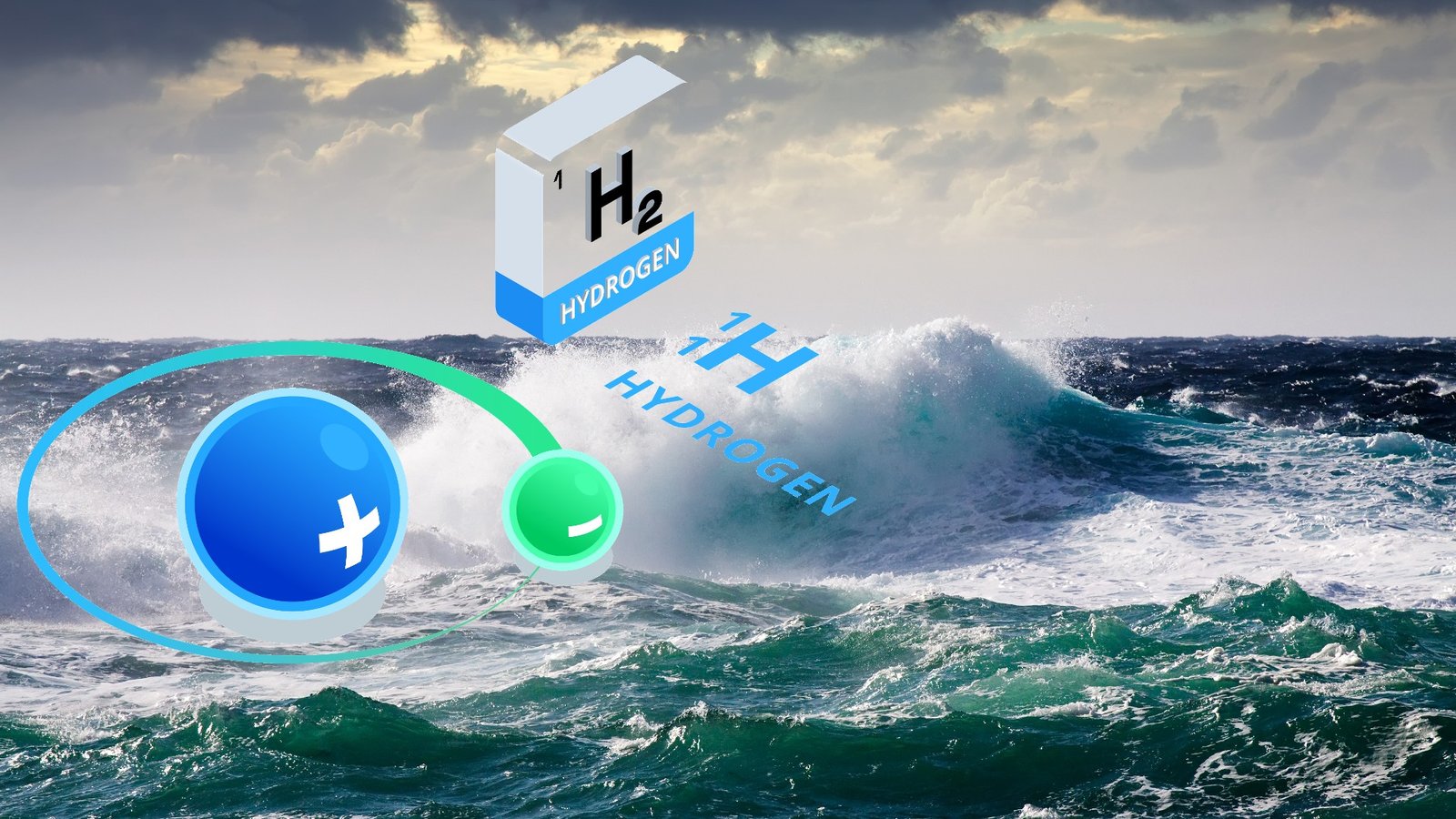
Researchers at the University of Adelaide have succeeded in producing hydrogen (H2) directly from seawater using inexpensive, abundant catalysts such as cobalt oxide with chromium oxide on its surface as the catalyst.

The researchers used a non-precious and inexpensive catalyst in a commercial electrolyzer to divide natural saltwater into oxygen and hydrogen with about 100% efficiency, allowing them to manufacture green hydrogen through electrolysis.
According to the University, saltwater is a nearly endless resource that may be used as a natural feed for electrolyte. This is more viable in areas with long beaches and plenty of sunlight.
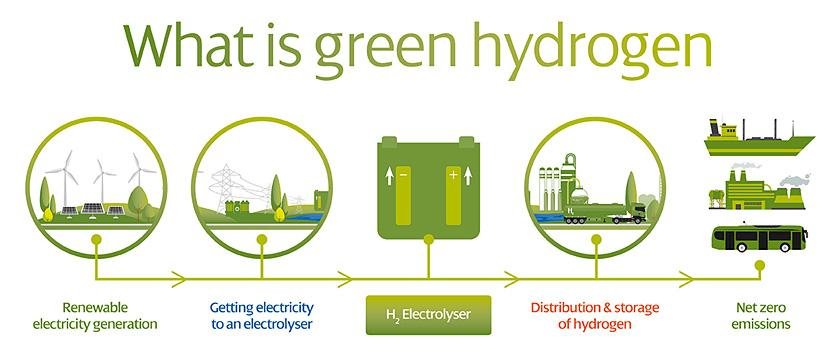
Because of electrode side reactions and corrosion caused by the complications of employing saltwater, seawater electrolysis is still in its early stages when compared to pure water electrolysis.
It is always necessary to treat impure water to a level of water purity for conventional electrolyzers including desalination and deionisation, which increases the operation and maintenance cost of the processes. This new hydrogen splitting solution presents a method for directly using saltwater without the use of pre-treatment systems or alkali addition, with performance comparable to that of existing metal-based mature pure water electrolysers.
Hydrogen from seawater is a pipe dream that may help to reduce the quantity of carbon dioxide blasted into the atmosphere every second of every day. This discovery holds the prospect of producing low-cost green H2 from sunlight and ocean. This hydrogen might reduce the carbon intensity of steel production, cement production, agriculture, and transportation.
Reference- Journal Nature Energy, Engadget, University of Adelaide PR, Clean Technica