Environmental researchers have unveiled a potentially game-changing discovery: a porous material capable of storing significant amounts of greenhouse gases. This development offers a powerful new tool in the fight against climate change.
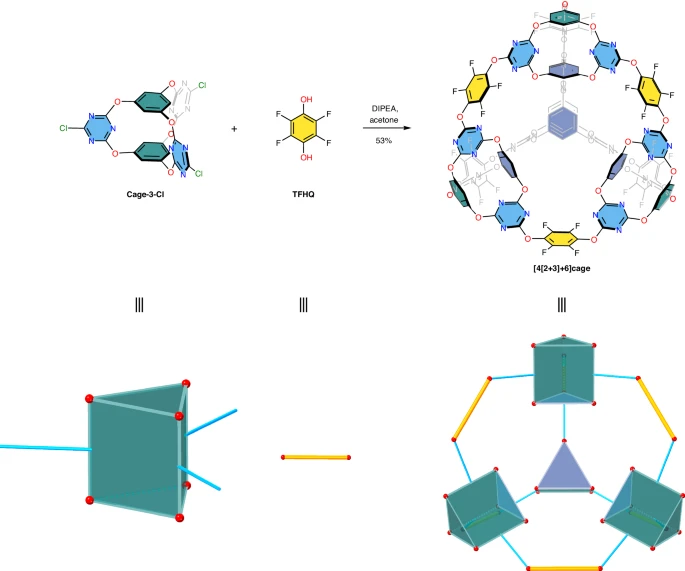
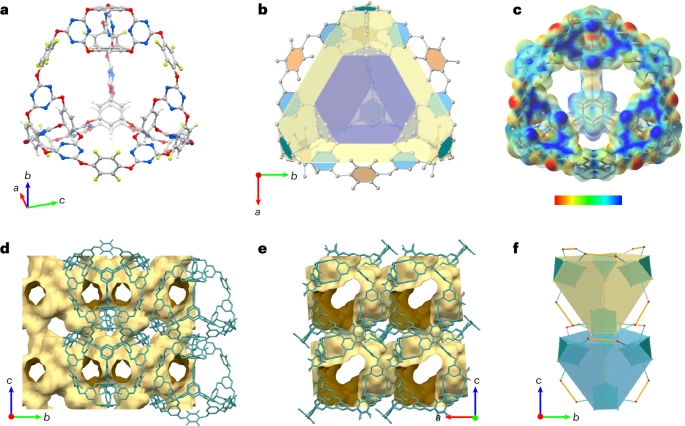
The research team, led by scientists from the UK’s Heriot-Watt University, utilized computational modeling to design the material. Their findings suggest its unique structure holds immense potential for capturing both carbon dioxide and sulphur hexafluoride, a particularly potent greenhouse gas.
“This discovery is a major step forward,” said Professor Marc Little, a lead researcher on the project. “The need for innovative porous materials is crucial in tackling the immense challenges posed by climate change.”

The newly developed material is an organic supermolecule, essentially microscopic cages formed by the combination of oxygen, nitrogen, and fluorine. This cage-like structure allows for the efficient capture and storage of greenhouse gas molecules.
“While tree planting remains a valuable method for carbon sequestration, its impact is slow-acting,” explains Professor Little. “This novel porous material offers a human-made solution capable of rapidly capturing greenhouse gases from the environment.”
Beyond this breakthrough, researchers are actively exploring other potential carbon capture solutions. These include boron-based two-dimensional structures with exceptional surface area, ideal for capturing emissions from power plants.
Additionally, scientists are investigating the modification of concrete, a significant contributor to greenhouse gas emissions due to cement production. Studies suggest that incorporating materials like baking soda into concrete could transform it into a net carbon absorber.
But the big hurdle is that many of these new materials are basically lab experiments. That’s a challenge for anybody who wants to use material science to tackle climate change — how do you make the leap from lab to market?
Reference- Journal Nature Synthesis, Heriot-Watt University, Interesting Engineering, Futurism






