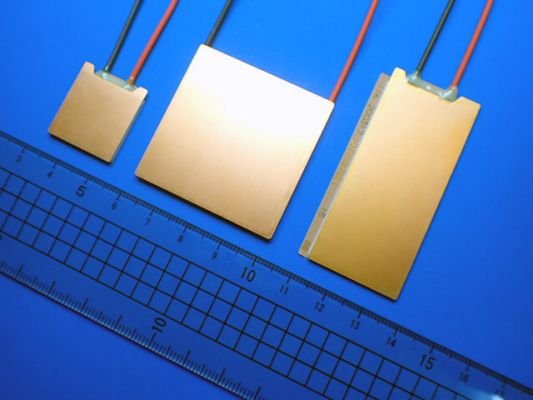
The need for a clean energy transition is obvious, pressing, and unavoidable. Energy efficiency is a component of the transition solution. Over 72% of all energy generated on a global scale is wasted as heat. For instance, a car’s engine consumes around 30% of the fuel it burns to propel the vehicle. The rest of the energy is lost as heat.
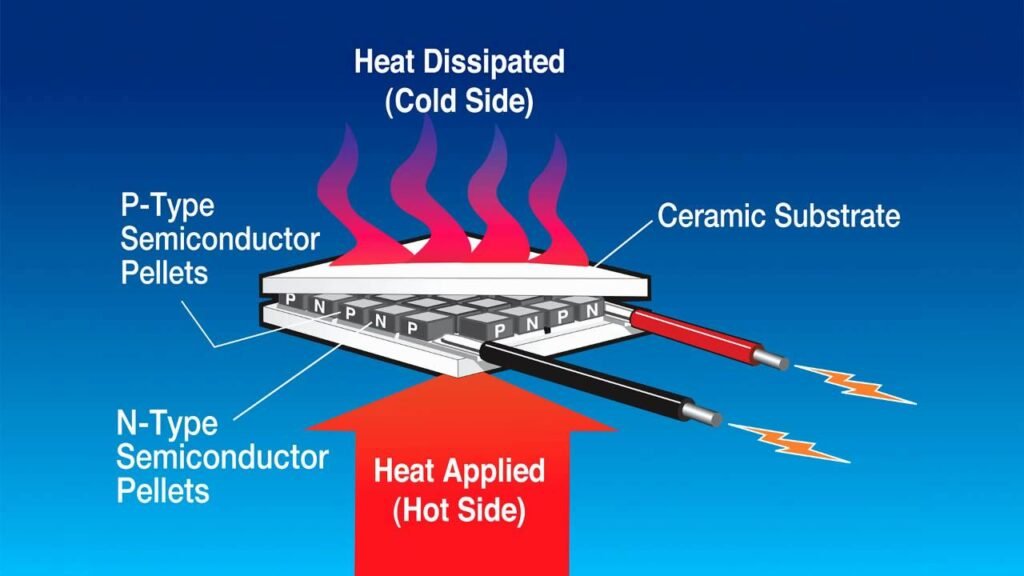
Even recovering a small percentage of this wasted energy would have a significant influence on climate change. Thermoelectric materials may assist by converting waste heat to usable power.

The thermoelectric materials convert heat to electrical energy through the “Seebeck effect.” Thomas Johann Seebeck, a German scientist, discovered in 1826 that exposing the ends of linked pieces of dissimilar metals to varying temperatures formed a magnetic field, which was eventually identified as being caused by an electric current.
Thermoelectric devices are used in a wide variety of applications today, from energy production in space probes to cooling devices in compact freezers. For instance, space adventures are propelled forward by radioisotope thermoelectric generators, which transform the heat generated by naturally decaying plutonium to energy.
Despite this broad range of uses, thermoelectric materials’ widespread commercialization is still constrained by their poor efficiency.
The optimal thermoelectric material would combine the electrical capabilities of semiconductors with glass’s low heat conductivity. However, this rare combination of features is not seen in natural materials.
A collaborative effort combining government-funded labs and universities in the United States, Canada, and Europe has identified over 500 previously unknown materials with a high anticipated thermoelectric efficiency. These first findings strongly imply that more calculations can determine the most effective material combinations for generating clean energy from waste heat and averting the global disaster that looms.
Reference- Nature Materials, McMaster University Article, Journal Of Materials Chemistry C